Table of Contents
Intro and history
Hcgenerate (N2Generate) is a natural testosterone booster, and comprehensive supplement, that was created about 10 years ago by Need To Build Muscle (n2bm). At the time there were many options for testosterone boosters, but you would have to purchase many different ingredients to get the best synergy. This is quite expensive. Luckily, the creators of Hcgenerate (N2Generate) came up with the perfect formula, updating it to the ‘Elite 8’ of ingredients in the past few years to make it even better.

Name change
Hcgenerate (N2Generate) was originally just named Hcgenerate. However, due to the confusion of credit card processors who rejected the sale of Hcg (Pregnyl) and to celebrate the n2bm brand’s popularity, the name was updated to N2generate. Many of the old school fans of the product refer to it as ‘the classic’ version of Hcgenerate. Have no worries though, the ingredients of Hcgenerate and N2generate are the same, and the product is as popular as it has ever been.
How is it different than other products?
If you walk into any supplement shop, or go online, you will find many different choices of testosterone boosters. In fact, I decided to look on GNC’s website and simply searched for ‘test boosters’ and came up with over 60 different products with dozens of brands. The prices ranged from $25 up to $200. When I looked at what the ingredients were in these products, I was blown away by why anyone would spend even $5 on them. One product, for example, contained a couple minerals like Zinc and a vitamin. However, because it had a big meathead flexing his muscles and a fancy name on the label, people decided to buy it. Another product was priced pretty high, and it did have some good ingredients like Fadogia and Fenugreek, but the dosages were pathetically low where they would barely do anything for you.
It is very important to always check labels and read the ingredients when choosing a supplement. Hcgenerate (N2Generate) has a rock solid label and 8 of the best ingredients (as mentioned earlier), working together in synergy, along with ample dosages.
Hcgenerate (N2Generate) is not your average testosterone booster, it is above and beyond anything else in its class. Like comparing a Ford to a Ferrari.
Why should you use it
Hcgenerate (N2Generate) will help you whether you are on a steroid cycle, during post cycle therapy, during bridge, or even solo. It will naturally boost your hormones, including testosterone, send your libido sky high, help prevent erectile dysfunction, build lean muscle mass with strength, and much more. The person who came up with the formula wasn’t some middle aged fat guy in a suit in a boardroom, he was an actual bodybuilder/athlete who wanted to create something that really worked for himself and his customers.
Ingredients and dosages
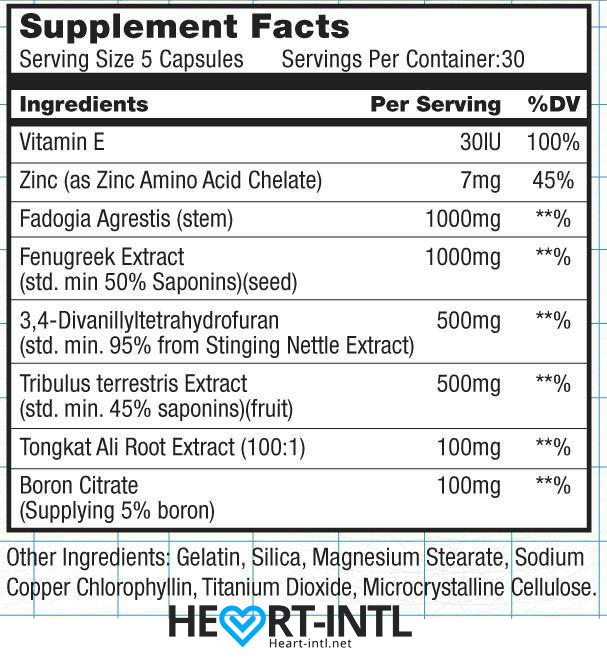 Each bottle of Hcgenerate (N2Generate) contains 30 servings and each serving is 5 capsules.
Each bottle of Hcgenerate (N2Generate) contains 30 servings and each serving is 5 capsules.
Let’s take a look at each of the 8 ingredients, with their dosages per serving, and examine what they each do:
Vitamin E 30IU:
Vitamin E is one of the most important vitamins for athletes. First, it is a potent antioxidant that helps keep you healthy. Second, it helps promote muscle repair and growth while inhibiting muscle breakdown. Finally, it is essential for your heart health, endurance, and eyes.
Zinc 7mg:
Athletes who have Zinc deficiencies will find themselves in big trouble. First, we know it speeds up chemical reactions in the body that boost muscle building. Next, it regulates hormones in the body. Those who have low testosterone usually also have poor Zinc levels. Finally, it increases protein synthesis during and after your workout which is crucial to get you much-needed results.
Fadogia Agrestis 1000mg:
It is no secret how popular Fadogia Agrestis has become over the past decade in the fitness community. It will help plump your testicles, raise testosterone, and boost libido. Fadogia should always be dosed at a strong amount plus be used as part of a stack. Have no worries, Hcgenerate (N2Generate) has plenty to do the job!
Fenugreek Extract 1000mg:
Popularly used during post cycle therapy, Fenugreek Extract works well at boosting sexual performance. It also has benefits for lipid health, especially cholesterol, and the kidneys. Next, studies have shown that men who used it had a rise in sexual desire (libido) by more than 30% after just 4 weeks. Finally, it has been given in folk medicine to help with ejaculation problems.
3,4 Divanillytahydrofuran (Divanil) 500mg:
One of the most underrated compounds in fitness. 3,4 Divanillytahydrofuran (Divanil) is very good at binding to SHBG (Sex hormone binding globulin), which means it will boost the ratio of free testosterone. This is an awesome thing not just during post cycle, but also on cycle, because it will boost your steroids power. Higher free testosterone means you gain muscle, gain strength, increase libido, and drop body fat much easier.
Tribulus Terrestris Extract 500mg:
Tribulus Terrestris is another one that is popularly used solo, but works so much better when stacked with other supplements. It contains steroidal saponins which increase hormones such as testosterone and dihydrotestosterone. This is great for libido boosting, but it also helps with other things like sleep problems and vitality.
Tongkat Ali Root Extract 100mg:
You should use Tongkat Ali Root for 2 main reasons. First, it boosts testosterone levels naturally. Second, it will help with infertility. This makes it a great option during post cycle therapy. Some of the other things it can help you with is muscle growth and energy.
Boron Citrate 100mg:
Boron Citrate is very important to metabolize minerals in the body. It also helps with bones, energy, and brain function. It will also help boost testosterone levels and help the body overcome problems during your cycle and post cycle.
Best way to use
On cycle:
Use Hcgenerate (N2Generate) with your steroid cycle because it will help with your lipids, boost free testosterone, and provide an overall wellness. The dosage is 2-3 capsules twice per day.
During post cycle:
It is very important to use Hcgenerate (N2Generate) during your post cycle therapy because it will help balance your hormones, boost libido, and prevent muscle/strength loss. I recommend 3 capsules 2-3X per day.
Stand-alone:
There are two ways to use it as a stand-alone. The first is on non-exercise days using 2 capsules twice a day. The second is on workout days or before sex, where you would take 4-5 capsules, 1-2 hours before activity.
Side effects
Hcgenerate (N2Generate) is completely natural and safe to use year round with no side effects to worry about.
 Where to buy
Where to buy
Hcgenerate (N2Generate) is sold at Amazon under Hcgenerate, and at n2bm.com as N2generate. For special deals please check out the n2bm facebook page or the evolutionary.org/elitefitness.com forums. It is a completely legal product and you can buy it by using PayPal or a credit card.
N2Generate Review
-
Boost Testosterone
-
Improve Libido
-
Prevent erectile dysfunction
-
Build lean muscle mass
-
Increase Strength




