 We hate to start this on a somber note. But we have to. If only you guys could see the things that we have to endure on e-mail.
We hate to start this on a somber note. But we have to. If only you guys could see the things that we have to endure on e-mail.
Just the other day, we got an email asking us about SR9009, now (fondly) called Stenabolic.
The guy wanted to use SR9009 cause he was afraid that Cardarine (GW-501516) was giving him gut cancer. We asked him about his dosing schedule with Cardarine and he was using a paltry 5mg a day.
And to make things worse, he thought that SR9009 is a SARM.
To the whole wide world, SR9009 is not a SARM and Cardarine will not give you cancer unless you are guzzling 100s of bottles a day.
To put things into perspective, here’s our official SR9009 review. You can read our Cardarine review over here.
Anybody who sends us an email with questions like the one above will be tracked down and force fed 100 mg of Cardarine a day for the rest of their lives.
Table of Contents
What is SR9009 Stenabolic?
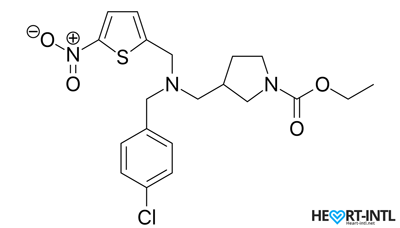
SR9009 (Stenabolic) Chemical Structure
SR9009 is a ligand or a molecule that attaches itself to another molecule, which in this case is a group of proteins called Rev-ErbA. By attaching itself to these, SR9009 amplifies their effects.
It was developed under Professor Thomas Buriss at the Florida campus of the Scripps Research Institute
The initial testing happened in mice and it gave them a tremendous endurance boost. They could run 50% longer than they used to and for a longer duration of time.
But that’s not where it gets interesting.
The rev-Erb-α gene in humans deals with a wide array of intracellular functions like metabolizing glucose and lipids, dealing with circadian rhythms and increasing the count of mitochondria in the cells.
All that translates into increased energy courtesy burning fat stores, enhanced metabolism and faster repair.
Would make an athlete drool, wouldn’t it?
That’s what makes SR9009 the blue-eyed baby of professional athletes and bodybuilders today.
How to use SR9009
One of the obvious advantages of using a compound like SR9009 is that you can use it in a standalone cycle or add it to your existing stack or steroid cycle. It blends in perfectly without any side effects whatsoever.
That’s because it is a non-hormonal compound. It will not shut you down or aromatize into estrogen (bitch tits). So, no PCT either.
Just cycle the drug for 8 weeks on and then 8 weeks off to avoid your body building tolerance to it.
Despite being an orally used compound, it is not toxic on your liver. However, as is the case with any other SARM or AAS, we highly recommend that you use liver supplements while you are on it.
The recommended dose is 20-30mg a day, depending on how your body adapts to the drug and your fitness goals.
However, SR9009 has one of the shortest half-life among bodybuilding supplements, about four hours. So, your daily dosage will have to be spaced out throughout the day to keep the levels of the drug stable in your body.
If you are using 20 mg in a day, use 5mg every 4 hours or so.
What can you expect after using SR9009?
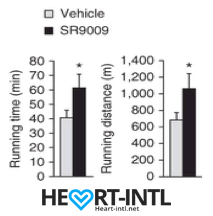
Running distance and time until exhaustion in endurance exercise in mice treated with SR9009
While you are on SR9009, think of it like your body is constantly exercising and recovering itself even when you are at rest.
- Supercharged metabolism: All those toxic shit labelled thermogenics that give you jitters and palpitations cannot amplify your metabolism like SR9009 does. It does it at an intracellular level and the effects are amazing.
- Fat Loss: By activating the metabolism of lipids and glucose, it burns more fat even when your body is resting. Clinical studies also indicate that SR9009 helps in reducing triglycerides and plasma glucose in the blood, which may make it one of the most effective treatments for conditions like Obesity and Type II diabetes.
- Endurance: SR9009 increases the number of macrophages in the cells which in turn leads to increased endurance. In the gym, you will be able to lift better. You will crush it in your cardio sessions even if you are doing it in a fasted state. To add to this, it also increases pro-inflammatory cytokine IL-6 by almost 72%. That’s reduced inflammation from strenuous exercise.
- Hypertrophy: Club this with a good diet and exercise routine and you get hypertrophy or bigger muscles.
Is it completely safe for use?
As of today, there are no known side effects for SR9009. However, if you notice anything untoward while using the compound, please stop using it immediately and speak to your healthcare provider about it.
To know how your body reacts to it, begin with a small dosage and gradually increase it to 20-30 mg a day.
Where can I buy authentic SR9009?
That’s the million-dollar question. Here’s a study that proves that the supplement industry for SARMS and other closely related compounds is rife with counterfeits. Most of these may contain banned hormones or Steroids.
You have to be really careful about what you buy and where you buy it from.
Here’s our official, trusted supplier for Stenabolic.
-
Increased Metabolism
-
Fat Loss Acceleration
-
Improved Endurance
-
Muscle hypertrophy




